Abstract
Introduction: Recent data suggest a suboptimal antibody response to COVID-19 vaccination in patients with hematological malignancies, especially under therapy with monoclonal antibodies targeting B-cells. Herein, we evaluated the development of neutralizing antibodies (NAbs) against SARS-CoV-2 in patients with chronic lymphocytic leukemia (CLL), Non-Hodgkin Lymphoma (NHL) and Hodgkin's Lymphoma (HL) after vaccination with the mRNA BNT162b2 vaccine, up to 50 days post their first vaccine dose.
Methods: This is a large prospective study (NCT04743388) evaluating the kinetics of anti-SARS-CoV-2 antibodies after COVID-19 vaccination in healthy subjects and patients with hematological malignancies. We report here the results in CLL, NHL and HL patients in comparison to age- and gender-matched controls who were vaccinated at the same time period (January to May 2021).
After vein puncture, the serum of both patients and controls was collected on day 1 (D1; before the first BNT162b2 dose), on day 22 (D22; before the second dose of the BNT162b2) and on day 50 (D50; 3 weeks post second dose of the BNT162b2). Serum was separated within 4 hours from blood collection and stored at -80°C until the day of measurement. NAbs against SARS-CoV-2 were measured using FDA approved methodology (ELISA, cPass™ SARS-CoV-2 NAbs Detection Kit; GenScript, Piscataway, NJ, USA) on the abovementioned timepoints. A NAb titer of at least 30% is considered as positive, according to manufacturer, whereas a NAb titer of at least 50% has been associated with clinically relevant viral inhibition [Walsh et al. N Engl J Med 2020, 383, 2439-50]. Samples of the same individual were measured in the same ELISA plate.
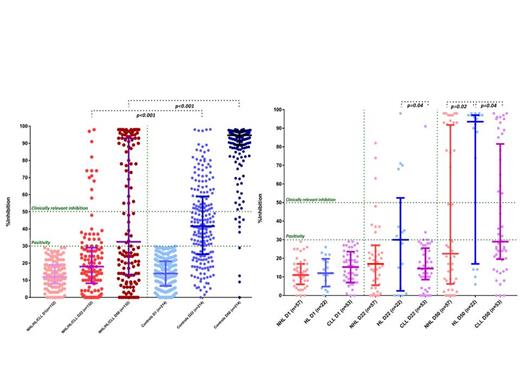
Results: We evaluated 132 patients with CLL/Lymphomas after vaccination with the BNT162b2. Patient population included 53 with CLL, 57 with NHL and 22 with HL, while 214 healthy controls, of similar age and gender, were also studied. At the time of vaccination, 30% (n=40) of patients had asymptomatic disease and out of 92 symptomatic patients, 49% (n=45) were on active treatment. Vaccination with two doses of the BNT162b2 led to lower production of NAbs against SARS-CoV-2 in patients compared with controls, both on day 22 and on day 50 (P<0.001 for all comparisons) for all subgroups. After the first dose of the vaccine, on D22, the patient group had lower NAb titers compared with controls: the median NAb inhibition titer was 18% (IQR: 8.5-29%) for patients versus 41.6% (IQR: 25.3-59%) for controls; p<0.001. On D50, the median NAb inhibition titer was 32.5% (IQR: 13.5-93%) for patients versus 94.7% (IQR: 89-97%) for controls; p<0.001. More specifically, only 50.8% (67/132) of the patients versus 98.1% (210/214) of the controls developed NAb titers ≥30% and 43.9% (58/132) of patients versus 95.3% (204/214) titers ≥50% (high protective titers) at day 50 (p<0.0001 for all comparisons; Figure-left part). Importantly, active treatment (which included anti-CD antibodies, Bruton's tyrosine kinase inhibitors, a combination of the above, chemotherapy-only regimens or Bcl-2 inhibitors) was an independent prognostic factor for suboptimal antibody response at day 50 (<50%) in the patient subgroup (p<0.001). Rituximab administration in the last 12 months correlated with decreased antibody response at day 50 (p<0.01). Patients with HL were more likely to achieve humoral responses (>50% at day 50) compared to other disease types (p<0.05; Figure-right part). Disease-related immune dysregulation and therapy-related immunosuppression were therefore involved in the low humoral responses seen in patients. Regarding adverse events, 9% and 9.8% patients reported mild reactions after the first and second dose of the BNT162b2 vaccine, respectively.
Conclusion: Patients with CLL/NHL/HL have a low humoral response following SARS-CoV-2 vaccination, particularly patients who are on active treatment with rituximab or BTK inhibitors. These patient subgroups therefore should continue utilizing protective measures against SARS-CoV-2 (masks, social distancing, etc) as they are at high risk for COVID-19. Further studies on the kinetics of immune subpopulations following COVID-19 vaccination will elucidate the underlying immune landscape and determine the potential need for additional booster vaccine doses or protective administration of antibodies against SARS-CoV-2 in CLL/NHL/HL patients with poor response after full vaccination.
Terpos: Sanofi: Consultancy, Honoraria, Research Funding; Novartis: Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Janssen-Cilag: Consultancy, Honoraria, Research Funding; GSK: Honoraria, Research Funding; Genesis: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; BMS: Honoraria; Amgen: Consultancy, Honoraria, Research Funding. Gavriatopoulou: Janssen: Honoraria; GSK: Honoraria; Genesis: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; Amgen: Honoraria; Karyopharm: Honoraria. Baltadakis: Amgen: Honoraria; Bristol-Myers Squibb: Honoraria; Alexion: Honoraria; Astellas: Honoraria; Pfizer: Honoraria, Other: Travel Grants; Gilead: Honoraria; Novartis: Honoraria; Abbvie: Honoraria; Genesis Pharma: Other: Travel Grants; Gilead: Other: Travel Grants; WinMedica: Other: Travel Grants; Baxalta Hellas: Other: Travel Grants. Dimopoulos: BMS: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Takeda: Honoraria; Beigene: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal